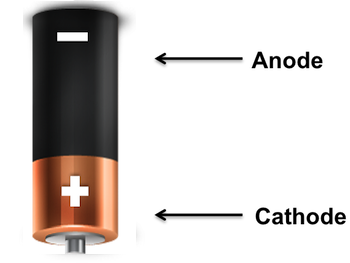
Battery
A battery is a device that stores chemical energy and converts it to electrical energy. The chemical reactions in a battery involve the flow of electrons from one material (electrode) to another, through an external circuit. The flow of electrons provides an electric current that can be used to do work. The role of a battery (or cell) in an electric circuit is to supply energy to the circuit by doing work upon the charge to move it from the low energy terminal to the high energy terminal. Historically, the word "battery" was used to describe a "series of similar objects grouped together to perform a function," as in a battery of artillery. In 1749, Benjamin Franklin first used the term to describe a series of capacitors he had linked together for his electricity experiments.
The average alkaline AAA, AA, C, D, 9-volt or button-cell battery is made of steel and a mix of zinc/manganese/potassium/graphite, with the remaining balance made up of paper and plastic. ... Being non-toxic materials, all of these battery “ingredients” are conveniently recyclable. A cell is a single unit device which converts chemical energy into electric energy. A battery usually consists of group of cells. Depending on the types of electrolytes used, a cell is either reserve, wet or dry types.
